Jose Rodriguez
University of California, Los Angeles
Talk Title
Solving Peptide Structures and More: Frontier Advances in Electron Diffraction
Presentation Time
SESSION 15: INNOVATIVE METHODS FOR PEPTIDE STRUCTURE
Thursday, June 29, 2023, at 03:20 pm - 03:45 pm
Electron diffraction has historically played an important role in the advancement of crystallographic approaches for the determination of complex small molecule structures. A broad array of atomic structures has now been determined by micro crystal electron diffraction, MicroED. They include naturally occurring peptides, synthetic protein fragments and peptide-based natural products. However, de novo structure determination by MicroED remains problematic for all but ideal crystals. Automated, fragment-based approaches to structure determination eliminate the need for atomic resolution diffraction, instead enforcing stereochemical constraints through libraries of small model fragments.
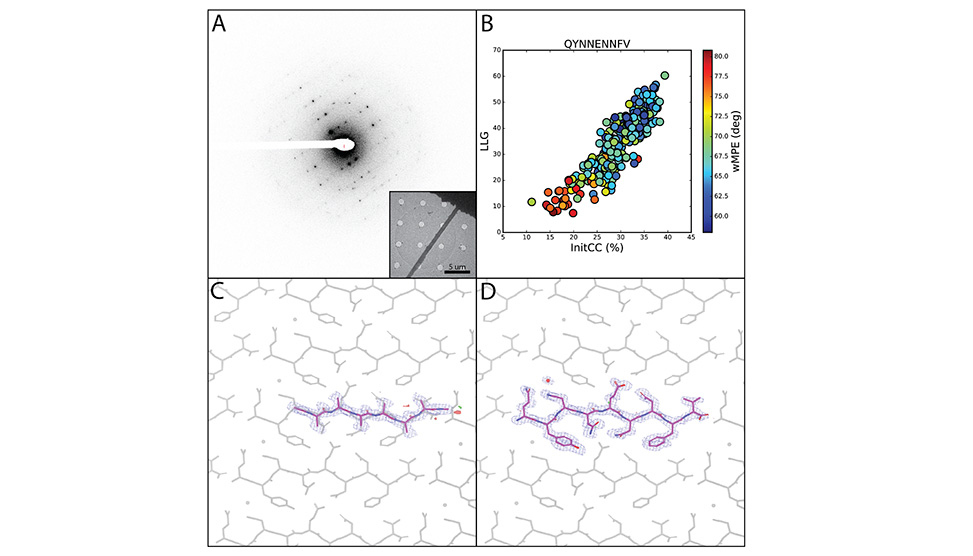
Determination of atomic resolution peptide and small molecule structures by frontier electron diffraction methods. Electron diffraction patterns, A, can be collected from nanoscale peptide crystals as inset, phased by fragment-based methods, B, and lead to accurate atomic structures, C and D.
We have demonstrated the application of fragment-based phasing on various macromolecular structures including some for which all traditional phasing methods have failed, see image above. Most recently, we have endeavored to determine structures where resolution is far beyond the reach of direct methods, yielding novel solutions with 1.4-2.2Å data. The determined structures are accurate and in test cases, reflect structures determined by traditional X-ray crystallographic approaches. New nanobeam electron diffraction methods are further advancing our ability to determine novel structures, by yielding atomic insights from regions of lattices containing as few as 1000 molecules. Together, these approaches advance the frontier of electron diffraction-based structure determination methods, and are increasingly accessible to the broader field of structural science.
Rodriguez joined the Department of Chemistry & Biochemistry as assistant professor in 2016 and holds the Howard Reiss Development Chair. His research group develops and applies new scientific methods in bio-imaging to solve cellular and molecular structures and reveal undiscovered structures that influence chemistry, biology and medicine. He conducts research on the complex architecture of biological systems – from single biomolecules to cellular assemblies – at high resolution. His research combines computational, biochemical and biophysical experiments.
Rodriguez is part of the team who have made profound discoveries through their use of electron microscopy, a field undergoing a revolution so significant that it was recognized through the 2017 Nobel Prize in Chemistry.
In 2020, Rodriguez spoke about his research and the importance of diversity and inclusion in the sciences in a Physical Sciences video, and he was recognized in Cell Press’ list of 100 inspiring Hispanic/Latinx scientists in America. He was also interviewed by science journalist Robyn Williams about his infectious rope-like prion proteins research.






