Zoë Adams
Scripps Research Institute
Talk Title
Stretching Peptides: Diyne-Braced Peptides as Beta-Strand Mimics
Presentation Time
Dr. Elizabeth Schram YI Oral Competition
Sunday, June 25, 2023 , at 12:00 pm – 12:15 pm
Extended amino acid backbone conformations are an abundant structural motif responsible for mediating a myriad of protein-protein interactions, PPIs. Yet, the shallow protein surfaces that dominate PPIs are challenging to target using standard methods and approaches for accessing extended backbone structures are limited. Often these types of interfaces include extended β-strand regions that define ordered backbone and side chain orientations that contribute to specific recognition of protein targets.
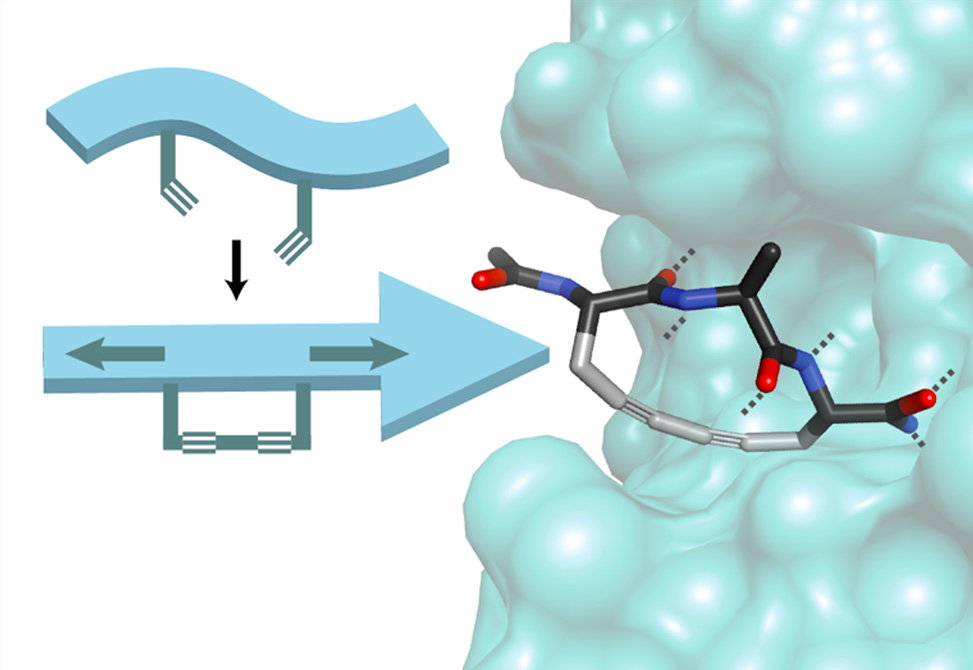
We explored the incorporation of a rigid, linear, diyne brace between side chains at the i to i+2 positions to generate a family of low-molecular-weight extended-backbone peptide macrocycles. NMR and DFT studies show that these stretched peptides adopt stable rigid conformations in solution and can be tuned to explore extended peptide conformational space.
This class of molecules is amenable to mimicking the backbone structure of natural β-strand motifs such as peptide ligands, inhibitors, and natural products by tuning the identity of the diyne macrocycle. The formation of the diyne brace is accomplished in excellent conversions, >95%, is amenable to high throughput synthesis, and the low molecular weight structure-inducing tripeptide core, <300 Da, is compatible with further synthetic elaboration. We showcase the utility of diyne-braced peptides with the synthesis of macrocyclic inhibitors of bacterial signal 1 peptidase.
Zoë Adams is a Ph.D. Candidate in the lab of Philip Dawson at Scripps Research Institute. Zoë received her B.S. in Chemical Biology from the University of California at Berkeley where she worked in the Matthew Francis lab, developing a screening system for selective metal chelating peptoid sequences. During her time in the Dawson lab, she has pursued structural and kinetic characterization and biological applications of extended peptides. These have included both diyne-braced peptide macrocycles and hydrogel competent peptides.






