Dennis Bong
The Ohio State University
Talk Title
Novel Fluorogenic Peptide Probes for Intracellular RNA and RNP Tracking
Presentation Time
SESSION 1: PEPTIDE TOOLS & PROBES
Sunday, June 25, 2023, at 09:45 am - 10:05 am
We introduce herein a new peptide-based strategy for intracellular RNA and DNA tracking that is robust, orthogonal, and complementary to existing methods: Fluorogenic U-Rich Internal Loop, FLURIL, tagging with cell-permeable bifacial Peptide Nucleic Acids, bPNAs.
Bifacial bPNAs utilize synthetic triazine bases to form triplex hybrid stems with oligo T/U domains that are biomimetic of native triple stranded structures formed from UAU base-triples. Our approach uses an 8-nt (U4xU4) U-rich internal loop, URIL, in the RNA of interest, ROI, as a compact labeling site for fluorogenic triplex hybridization with a bPNA probe, ̴1 kD, that derives from a tripeptide.
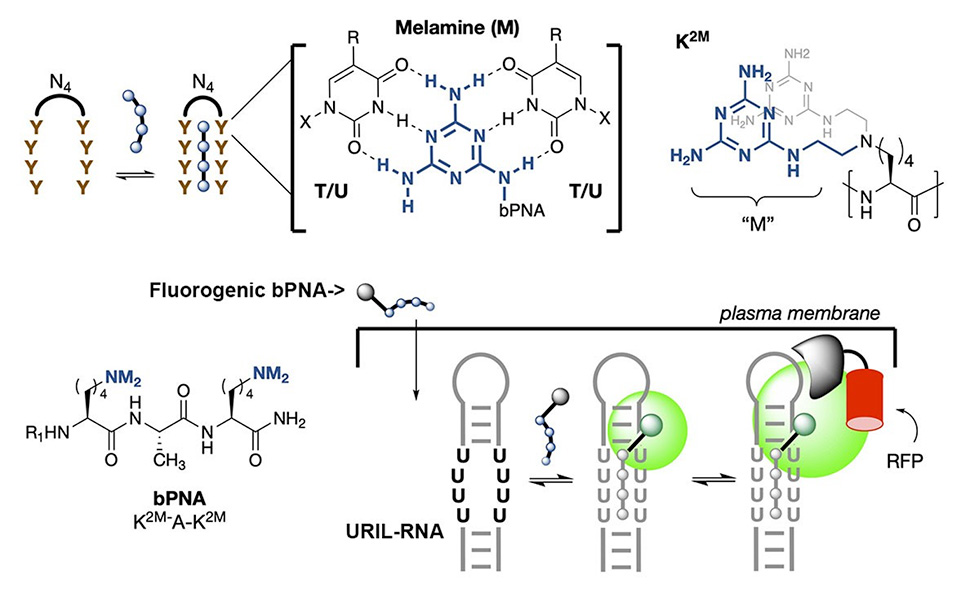
FLURIL tagging thus replaces a 4 bp duplex stem with a labeled 4-base-triple hybrid stem of similar structure and mass. In contrast to existing strategies for RNA tracking, FLURIL tagging can be applied to internal, genetically encoded URIL RNA sites with minimal structural perturbation, co-expression of protein-fusion labels, or significant increase in molecular weight and steric bulk. Together, these experiments show that FLURIL tagging can simply and reliably track intracellular RNA, RNPs, and DNA, with a streamlined molecular footprint relative to other methods. Our data also indicate that FLURIL tagging is fully compatible with existing labeling methods and thus may be used to broaden the scope of intracellular RNA and DNA tracking.
We further discuss structure-function studies that examine the impact of peptide structure on DNA/RNA binding by bPNAs. We find that there are two bPNA backbone modifications that significantly improve hybridization: alternating (D, L) configuration in open chain dipeptides and homochiral dipeptide cyclization to the diketopiperazine. The resulting family of di and tripeptides that efficiently bind T/U loops in nucleic acids is remarkably compact. We anticipate that the chemical tunability of this simple peptide probe scaffold will enable a broad range of applications for URIL-tagged intracellular RNA and RNP interactions.
Dennis Bong received a BSc in chemistry from the University of California at Berkeley while working with Professor K. Peter C. Vollhardt and his PhD from The Scripps Research Institute with Professor M. Reza Ghadiri. Following a postdoctoral stint with Professor Ronald Breslow at Columbia University in New York, he joined the chemistry faculty at The Ohio State University in 2004 where he is a full professor in the Department of Chemistry & Biochemistry.
Our research group uses organic synthesis and biochemistry to study the chemical biology of nucleic acids. Through synthesis and design, our group develops new functional polymer, peptide, lipid and nucleic acid assemblies to provide chemical solutions for problems in delivery and to develop novel compounds that can report on and modulate nucleic acid function. Though each research effort is grounded in organic synthesis, these synthetic systems are interrogated using biophysical, biochemical and cell culture methods.
Much of our current research centers on the use of an unnatural triazine heterocycle, melamine, as an artificial base triple with U/U or T/T. When displayed multivalently, the melamine base engages with two Watson-Crick faces of thymine/uracil in DNA/RNA, resulting in the formation of triple-stranded hybrid structures. Synthetic triplex hybridizing molecules that utilize melamine as a base triple are in a family of molecules we call “bPNA” to represent bifacial [Peptide/Peptoid/Polymer] nucleic acid, according to the backbone used for melamine display. We have studied bPNAs as allosteric switches for aptamer binding and ribozyme catalysis, functional probes, directed cleavage reagents as well as packaging and targeting elements for nucleic acid delivery. Current research continues along these lines.






