Tom N. Grossmann
VU University Amsterdam
Talk Title
From Protein Structures to Functional Biomimetics
Presentation Time
SESSION 3: NOVEL MODULATORS OF BIOLOGY
Sunday, June 25, 2023, at 02:15 pm - 02:40 pm
The inhibition of disease-relevant protein-protein interactions, PPI, represents an appealing strategy towards the development of novel therapeutics. Due to the extended nature of involved interaction areas, conventional ligand-discovery approaches that rely on small molecular scaffolds often fail to provide potent PPI inhibitors.1 Due to their excellent surface recognition properties, peptido- and proteomimetic molecules provide appealing alternatives.1, 2
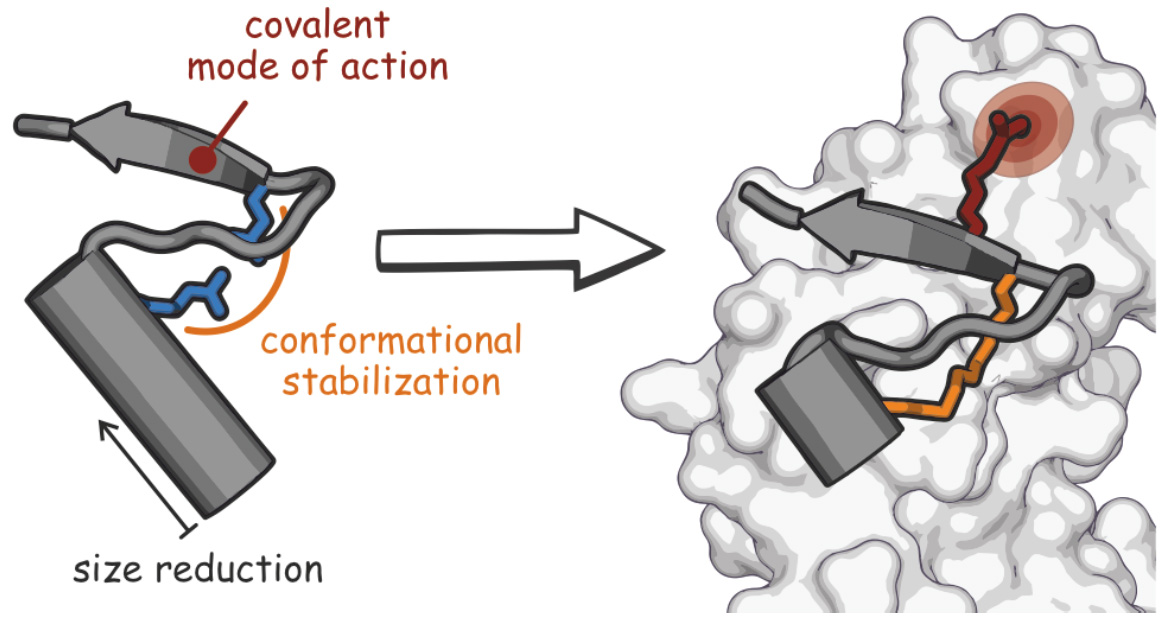
Testing a new anti-microbial strategy, we designed a proteomimetic derived from the peri- plasmic region of a bacterial protein, FtsB, that is part of the divisome complex.3 The bioactive conformation of this peptide was stabilized by a customized cross-link resulting in a tertiary structure mimetic with increased affinity for a FtsB binding partner, FtsQ. To increase activity, a covalent handle was incorporated, providing an inhibitor that impedes the interaction between FtsQ and FtsB irreversibly. The covalent inhibitor reduced the growth of an outer membrane-permeable E. coli strain, and affected the infection of zebrafish larvae.3 This first-in-class inhibitor of a divisome PPI highlights the potential of proteomimetics as inhibitors of challenging therapeutic targets.
References
1. Pelay-Gimeno, A. Glas, O. Koch, and T.N. Grossmann. Structure-Based Design of Inhibitors of Protein–Protein Interactions: Mimicking Peptide Binding Epitopes. Angew. Chem. Int. Ed. 2015, 54, 13, 8896–8927
2. W.S. Horne, and T.N. Grossmann. Proteomimetics as Protein-Inspired Scaffolds with Defined Tertiary Folding Patterns. Nature Chem. 2020, 12, 331–337
3. F.M. Paulussen, G.K. Schouten, C. Moertl, J. Verheul, I. Hoekstra, G.M. Koningstein, G.H. Hutchins, A. Alkir, R.A. Luirink, D.P. Geerke, P. van Ulsen, T. den Blaauwen, J. Luirink, and T.N. Grossmann. Covalent Proteomimetic Inhibitor of the Bacterial FtsQB Divisome Complex. J. Am. Chem. Soc. 2022, 144, 33, 15303–15313
Research in the Grossmann group centers around the synthesis of peptide-derived molecules and the engineering of proteins using organic reactions. Central aspects are the synthesis of non-natural amino acids as well as modified peptides, and the use of biocompatible reactions for the selective functionalization of unprotected peptides and proteins. For example, the group uses these techniques for the development of novel bioactive peptidomimetics and for chemical protein/enzyme engineering.






