Christina Schroeder
Genentech
Talk Title
Recifin A, a Novel and Selective Allosteric Inhibitor of Tyrosyl–DNA Phosphodiesterase I with a Unique Disulfide-bond Topology
Presentation Time
SESSION 7: FROM NATURAL PRODUCTS TO UNNATURAL BIOACTIVE PEPTIDES
Tuesday, June 27, 2023, at 09:40 am - 10:00 am
Tyrosyl-DNA phosphodiesterase 1, TDP1, is a molecular target for the sensitization of cancer cells to the FDA-approved topoisomerase inhibitors topotecan and irinotecan. However, current TDP1 inhibitors have low binding affinity or are substrate mimics with low specificity. Through high-throughput screening of natural products and extracts library in the search for novel TDP1 inhibitors, we identified a new class of complex knotted peptides with a unique disulfide-bond topology from the marine sponge Axinella sp.1
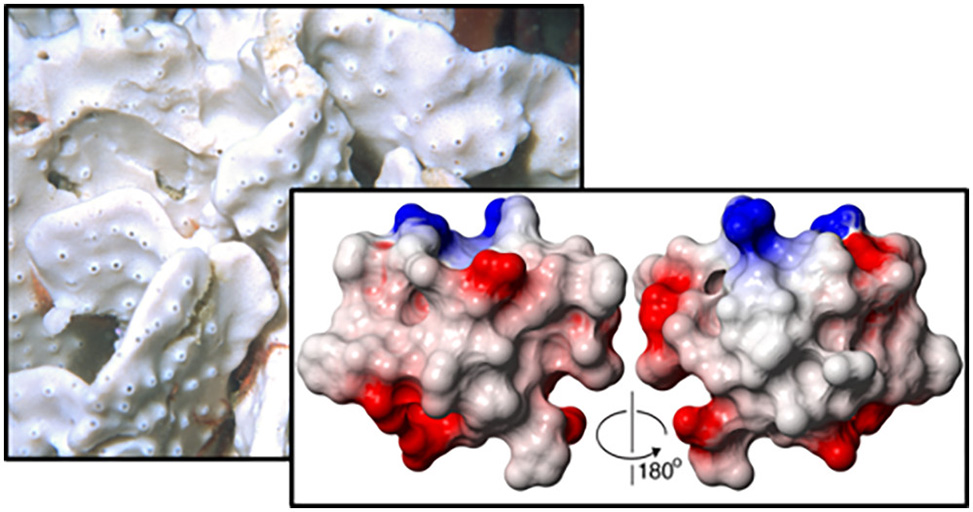
The active component was a 42-residue peptide named recifin A. Unlike previously described TDP1 inhibitors which bind to the C-terminal catalytic domain of TDP1, recifin A acts as an allosteric inhibitor and binds to the N-terminal regulatory domain. The three-dimensional NMR structure revealed a novel fold comprising a four-strand antiparallel }-sheet and two helical turns stabilized by a complex disulfide-bond network that creates an embedded ring around one of the strands. The structure is locked in place by a centrally located tyrosine residue, resulting in the Tyr-lock family name.
Recifin A represents both the first of a unique structural class of knotted disulfide-rich peptides and defines a previously unseen mechanism of TDP1 inhibition that could lead to the development of a new class of TDP1 inhibitors with improved specificity that could be exploited for potential anticancer applications.
Reference
1. Lauren R. H. Krumpe, Brice A. P. Wilson, Christophe Marchand, Suthananda N. Sunassee, Alun Bermingham, Wenjie Wang, Edmund Price, Tad Guszczynski, James A. Kelley, Kirk R. Gustafson, Yves Pommier, K. Johan Rosengren, Christina I. Schroeder, and Barry R. O'Keefe. Recifin A, Initial Example of the Tyr-Lock Peptide Structural Family, Is a Selective Allosteric Inhibitor of Tyrosyl-DNA Phosphodiesterase I. JACS, 2020, 142, 21178-21188.
Associate Professor Christina Schroeder is a bioactive peptide engineer who uses venom-derived peptides from spiders, cone snails and snakes to develop novel treatments for chronic and neuropathic pain. She is particularly fascinated by the possibility of harnessing the venom from an animal that has evolved to kill its prey to develop something that could benefit human kind.
The ultimate result of Christina's research is to develop a treatment that allows people to manage chronic pain, a condition that one out of five Australians suffers from, and which currently has inadequate treatments. To that end, she is exploring the relationship between drugs and receptors, focusing on expanding on the traditional lock and key mechanism to include the membrane surrounding the receptors. Christina aims to unlock a detailed understanding of how these venom-derived peptides engage with receptors in the body and how we can use this knowledge to design more potent drugs with fewer side effects.






